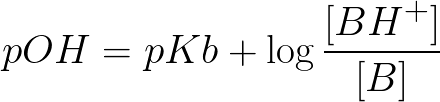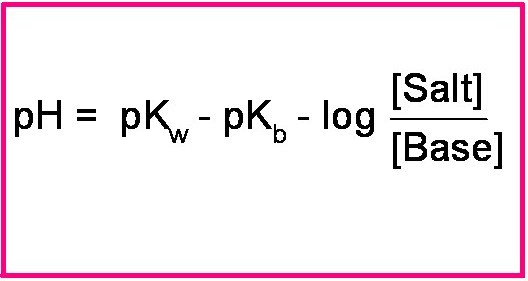Henderson Hasselbalch Equation Pkb
The henderson hasselbalch equation is.
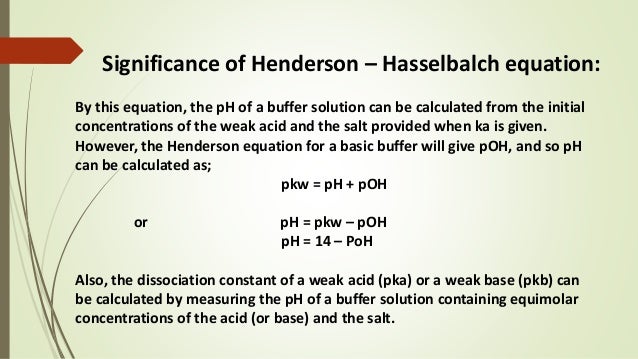
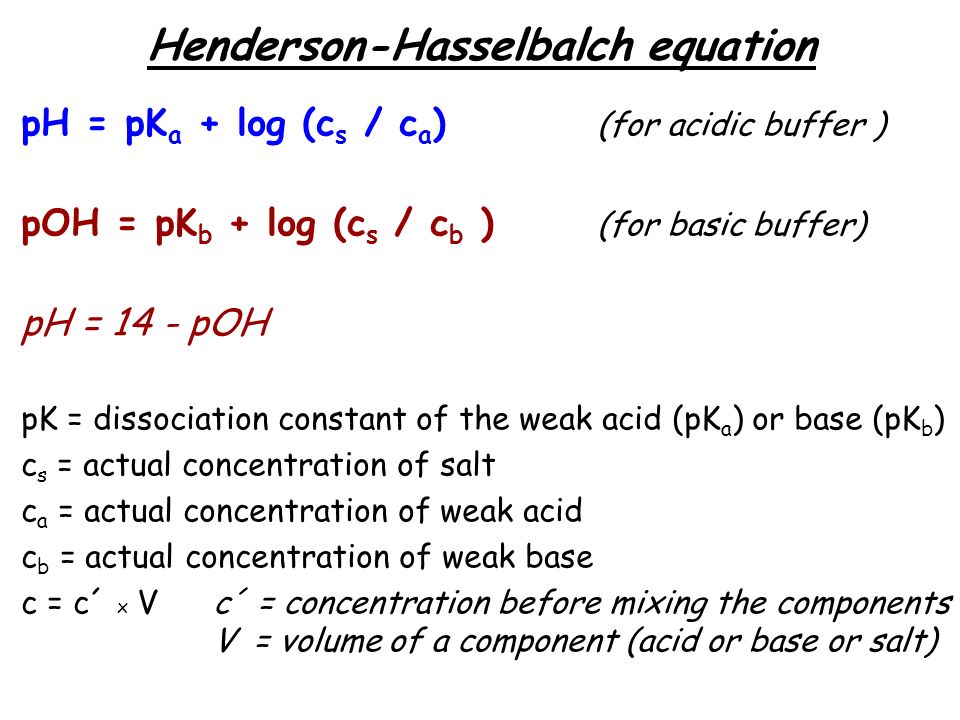
Henderson hasselbalch equation pkb. A buffer is a solution which can resist the change in ph. Chemically a buffer is a solution of equimolar concentration of a weak acid such as acetic acid ch 3 cooh and its conjugate base such as acetate ion ch 3 coo. Ammonia nh 3 is always a base and the ammonium ion nh 4 is the conjugate acid of ammonia. Note that here ch 3 cooh ca and ch 3 coona cb this equation is also known as henderson hasselbalch equation.
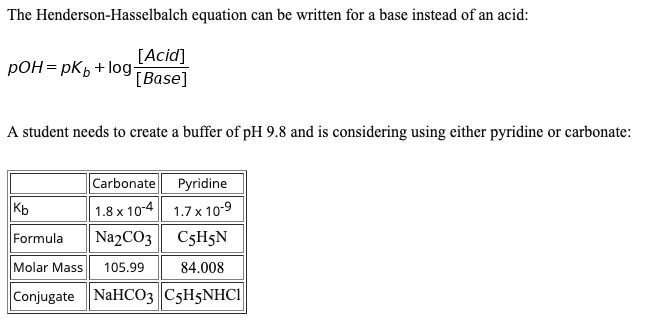
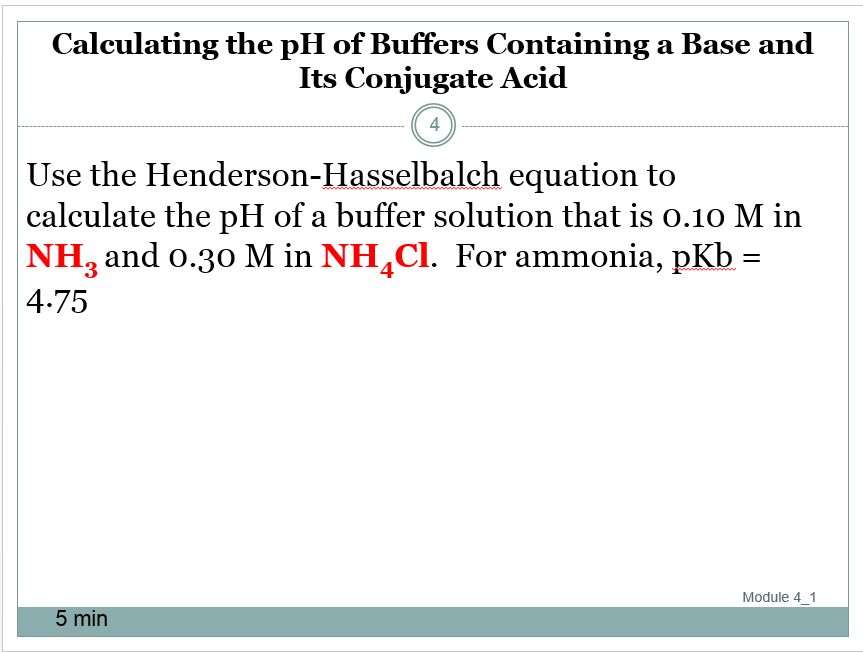
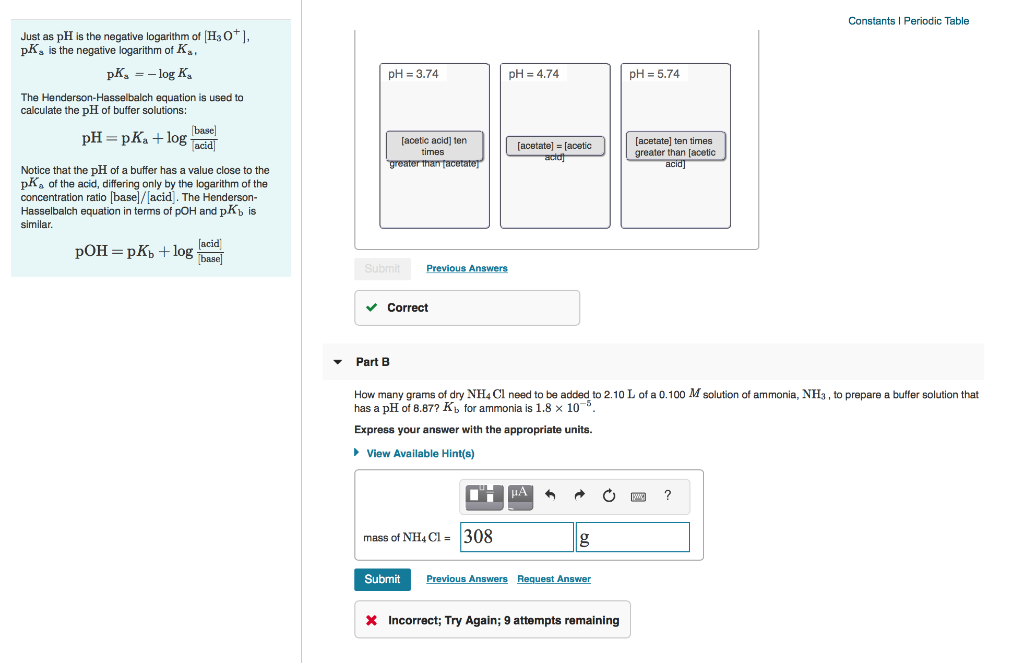
The reason the henderson hasselbalch equation is an approximation is because it takes water chemistry out of the equation. Example problem applying the henderson hasselbalch equation. Now we can use this equation. Calculate the ph of a buffer solution made from 0 20 m hc 2 h 3 o 2 and 0 50 m c 2 h 3 o 2 that has an acid dissociation constant for hc 2 h 3 o 2 of 1 8 x 10 5.
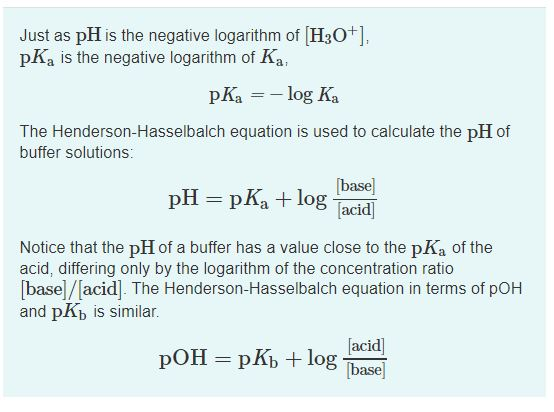
The henderson hasselbalch equation also describes the characteristic shape of the titration curve of any weak acid such as acetic acid phosphoric acid or any amino acid. Assumptions for the henderson hasselbalch equation. Henderson hasselbalch equation is a simple expression which relates the ph pka and the buffer action of a weak acid and its conjugate base. In chemistry and biochemistry the henderson hasselbalch equation can be used to estimate the ph of a buffer solution the numerical value of the acid dissociation constant k a of the acid is known or assumed the ph is calculated for given values of the concentrations of the acid ha and of a salt ma of its conjugate base a.
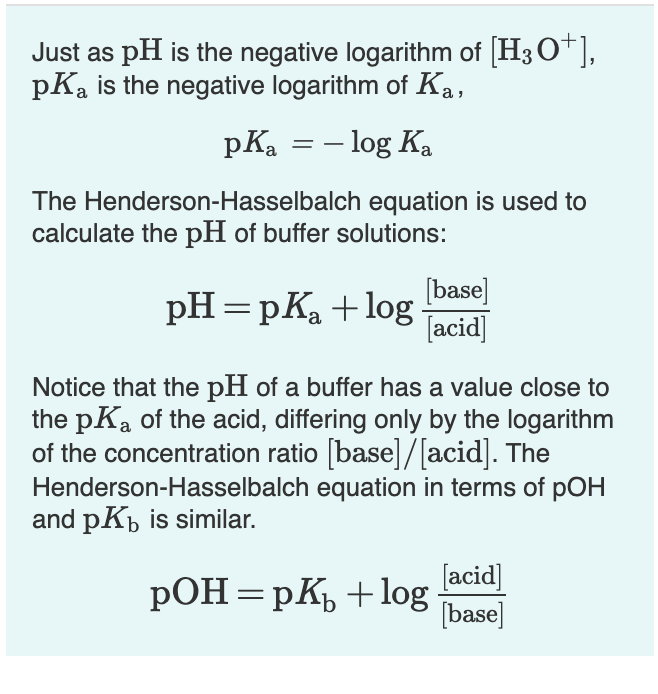
Henderson hasselbalch equation is a numerical expression which relates the ph pka and buffer action of a buffer. The derivation of the henderson hasselbalch equation is therefore significant for any student of pharmaceutical sciences. The henderson hasselbalch equation is extremely useful in correlating ph with pka and concentrations of unionized and ionized species. 1 a rearrangement of the k a expression followed by 2 the use of negative logarithms.
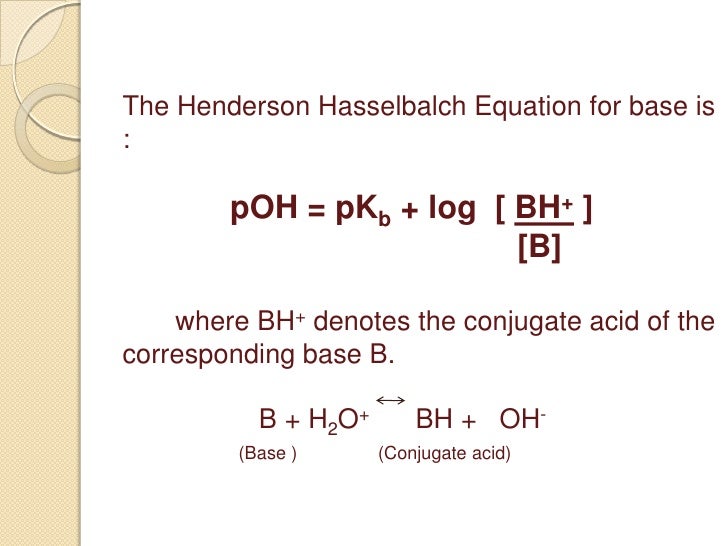
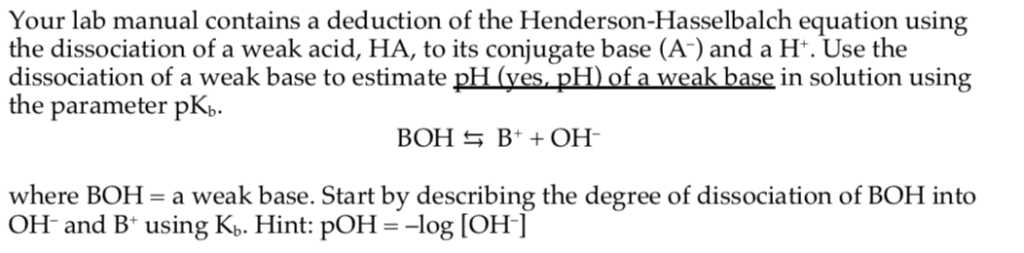
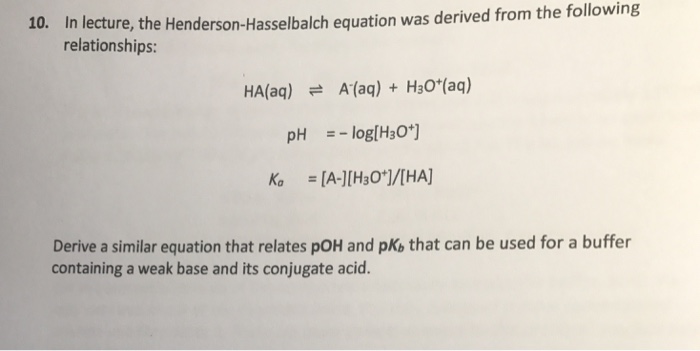
Return to the acid base menu. A conjugate acid has one more proton h than the base you started with. As you can see we are given a pkb instead of a pka. How to derive henderson hasselbalch equation.
Henderson hasselbalch equation for base buffer boh b 1 oh 1 kb b oh boh take log for each side of equation log kb log oh log b boh p log pkb poh log b boh poh pkb log salt base henderson hasselbalch equation for base buffer use henderson hasselbalch equation to determine ph for 0 80m hf 0 50m naf. That buffer solution has a ph of 9 65 before i introduce henderson hasselbalch s equation we should identify the acid and base. For example the solution may contain.